- Home
- Hip Implant
Hip Implant
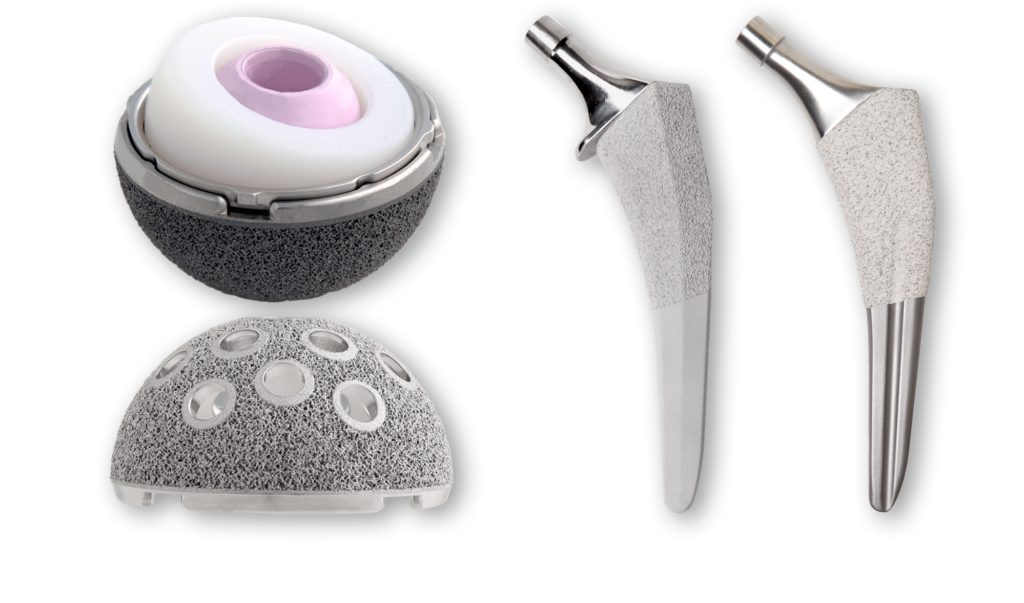
General Information about Hip Implants
Hip implants are medical devices intended to restore mobility and relieve pain usually associated with arthritis and other hip diseases or injuries. Every hip implant has benefits and risks. Every hip implant system has unique device design features such as size, shape, and material, and dimensions. In addition, the same hip implant system may have different outcomes in different patients. There are several factors that may influence the outcome and longevity of a hip implant including the device design features, surgeon experience and implantation technique, and individual patient characteristics such as age, sex, weight, activity level and overall health.
Although hip replacement is typically successful, it is important to know that hip implants may need to be replaced eventually.
Materials Used in Hip Implants
In the United States, there are currently four types of total hip replacement devices available with different bearing surfaces. These are:
- Metal-on-Polyethylene: The ball is made of metal and the socket is made of plastic (polyethylene) or has a plastic lining.
- Ceramic-on-Polyethylene: The ball is made of ceramic and the socket is made of plastic (polyethylene) or has a plastic lining.
- Ceramic-on-Ceramic: The ball is made of ceramic and the socket has a ceramic lining.
- Ceramic-on-Metal: The ball is made of ceramic and the socket has a metal lining.
An orthopaedic surgeon should determine which hip implant will offer the most benefit and least risk for each patient.
As of May 16, 2016, the effective date of the final order requiring premarket approval applications for these devices, there are no FDA-approved metal-on-metal total hip replacement devices marketed for use in the US. However, there are some patients who received a metal-on-metal total hip replacement prior to May 16, 2016. The FDA’s Metal-on-Metal Total Hip Replacement Implant webpage provides specific information on metal-on-metal total hip replacements.
Free Case Review
Get in touch with a legal expert today. The info you provide will remain private, and never be shared without consent.
Get Legal Help
Get In Touch
Call or Text 24/7
